Cardiac Arrhythmia: An Ominous Side Effect of Thrice-weekly Hemodialysis?
Many studies represent incremental gains in our understanding of human pathophysiology. Some studies, especially large randomized clinical trials, can singlehandedly change the standard of care. Other studies, at the most unexpected times, flash a signal that raises the question of whether the widely accepted standard of care is simply inadequate.
In the April 2018 issue of Kidney International, Prabir Roy-Chaudhury and colleagues published one such study. These investigators reported data from a prospective, multicenter study, the Monitoring in Dialysis (MiD) Study, which was conducted in both the United States and India and aimed to estimate the proportion of thrice-weekly hemodialysis patients who experienced clinically significant arrhythmias during 6 months of follow-up.1 The occurrence of arrhythmia was identified with implantable, continuous cardiac monitoring devices (Reveal XT or Reveal LINQ, which are manufactured by Medtronic).
Clinically significant arrhythmias were defined prospectively:1
- Ventricular tachycardia ≥130 beats per minute for ≥30 seconds
- Bradycardia ≤40 beats per minute for ≥6 seconds
- Asystole for ≥3 seconds
- Symptomatic events with electrocardiography-confirmed arrhythmia
The study enrolled 66 patients; 62 (94%) completed 6 months of follow-up and the devices also detected atrial fibrillation.
2/3 patients experienced clinically significant arrhythmias2
The incidence rate of clinically significant arrhythmias was 4.5 events per patient-month. However, ventricular tachycardia was not the primary culprit. Indeed, only one such event occurred during all of follow-up. Instead, the leading arrhythmia was bradycardia, which occurred at a rate of 3.9 events per patient-month. Asystole occurred 14 times among 6 patients.
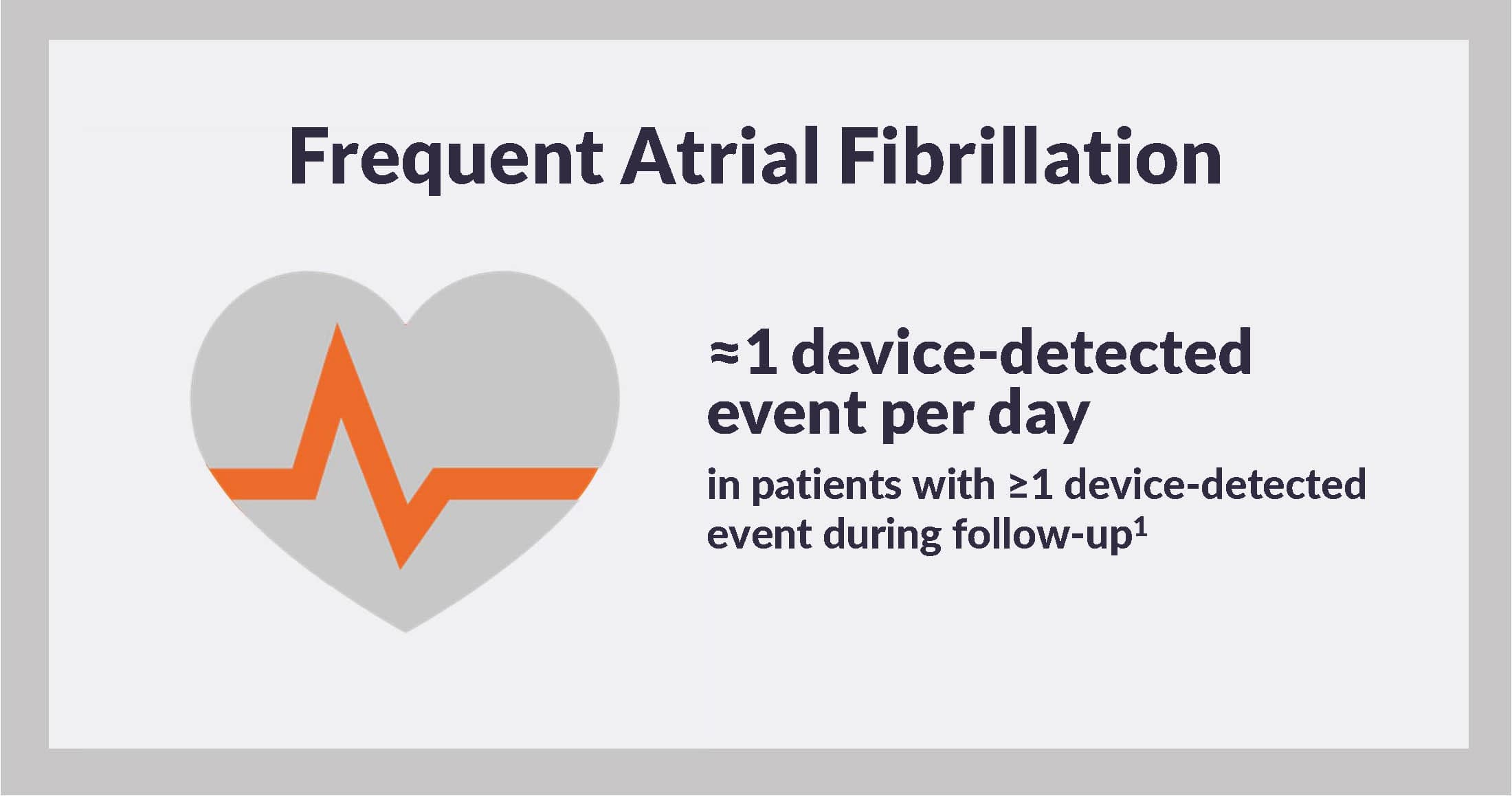
In addition, the incidence rate of device-detected atrial fibrillation was 11.9 events per patient-month, although events were concentrated among only 27 (41%) patients. Simple arithmetic shows that among patients with ≥1 device-detected atrial fibrillation, the incidence rate was equal to nearly 1 event per patient-day.
Timing of Clinically Significant Events2
From the perspective of potential iatrogenicity, the timing of arrhythmias is most ominous.
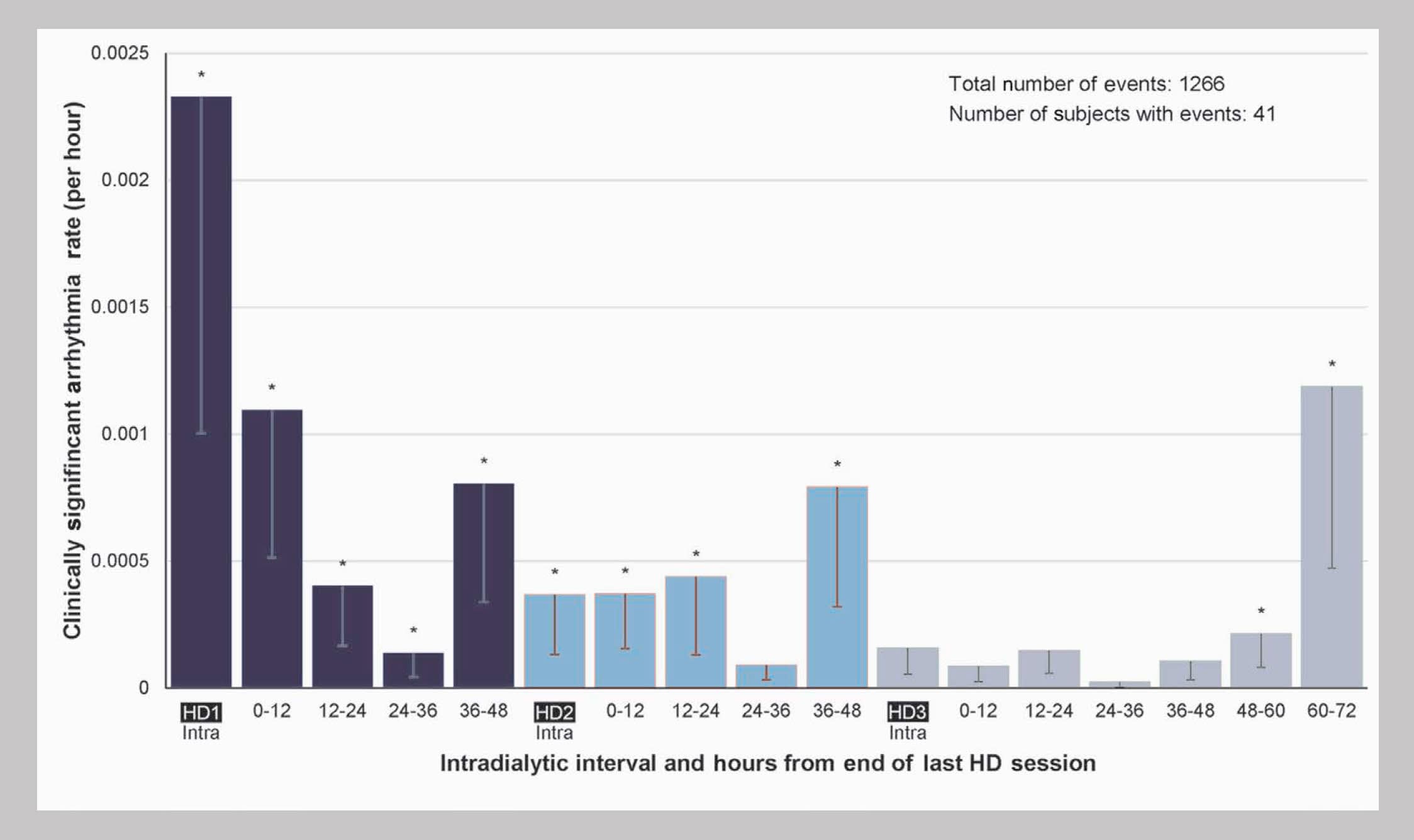
Incidence was highest during the first hemodialysis session of the week, second-highest during the final 12 hours of the long interdialytic gap, third-highest during the 12 hours immediately following the first session of the week, and meaningfully elevated during the final 12 hours of each of the short interdialytic gaps.
For bradycardia, incidence was highest during the final 12 hours of the long interdialytic gap, second-highest during the final 12 hours before the last hemodialysis session of the week, and third-highest during the final 12 hours before the second session of the week.
The incidence of device-detected atrial fibrillation was highest during hemodialysis sessions.
Some parameters were significantly different between sessions with and without arrhythmias:2
Parameters during sessions with and without clinically significant arrhythmias were compared.
In sessions with arrhythmias:
- Dialysate temperature was more likely to be ≥37℃
- Dialysate calcium was more likely to be 2.5 mEq/L
- Sodium modeling was likely to be employed
Bradycardia and asystole were likely to occur on days during which patients did not take either beta blockers or non-dihydropyridine calcium channel blockers (e.g., diltiazem and verapamil).
The incidence of clinically significant arrhythmias was associated with increased risk of cardiovascular hospitalization.2
Specifically, a clinically significant arrhythmia during the past 14 days was associated with 8.8-fold risk of cardiovascular hospitalization! However, during the 6 months of follow-up, no sudden deaths occurred.
The high frequency of clinically significant arrhythmias and device-detected atrial fibrillation is startling, but offers a needed explanation for the high incidence of sudden death and stroke in the dialysis patient population.
For years, the United States Renal Data System has reported that arrhythmia and cardiac arrest comprise the leading causes of death on dialysis.3
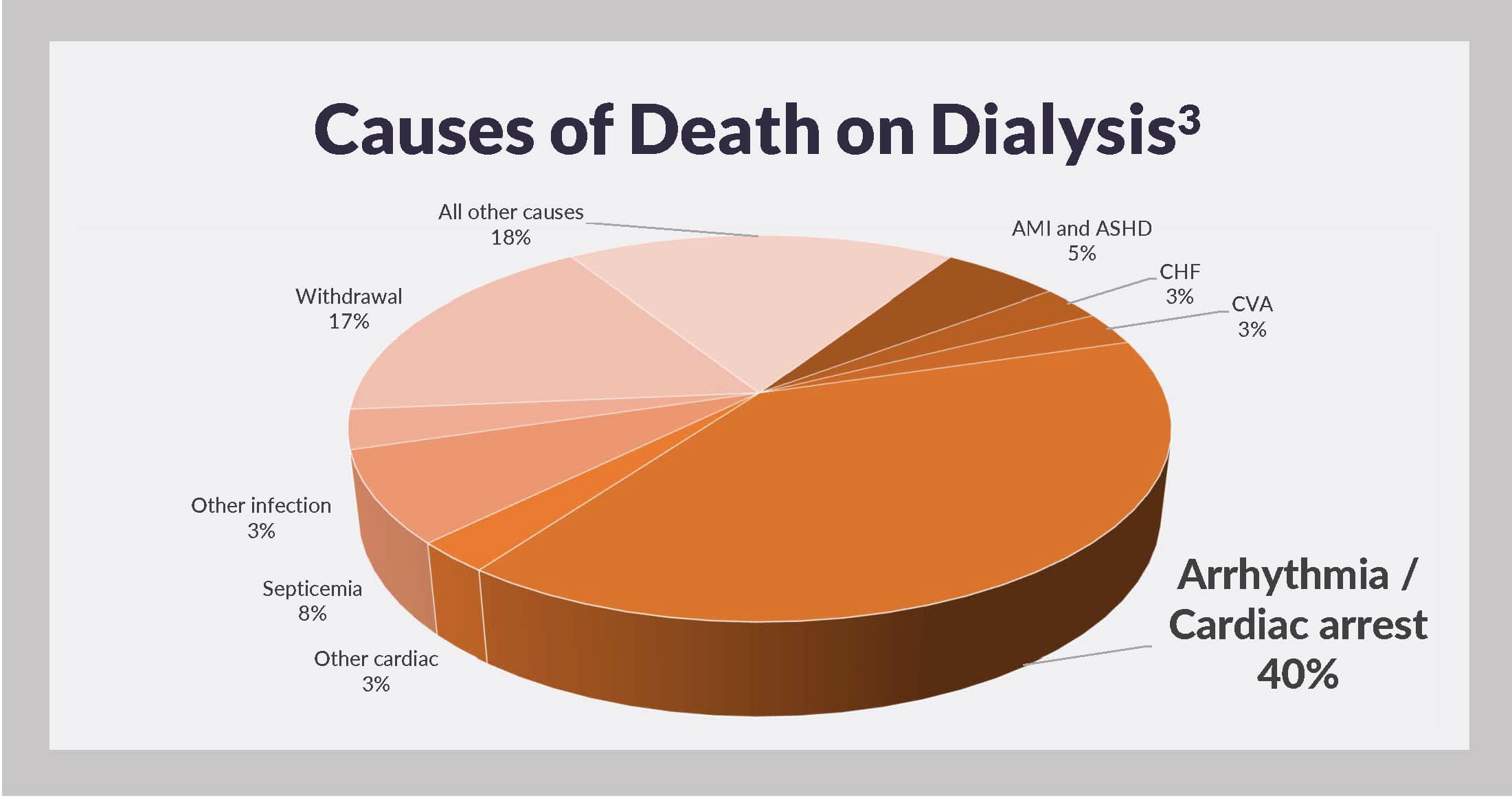
In 2014, these causes were collectively responsible for 40% of dialysis patient deaths with a known cause and 29% of all deaths, including those due to unknown causes. Risk of stroke, a potentially direct consequence of atrial fibrillation, is also higher among hemodialysis patients, relative to general population members.
And what about thrice-weekly hemodialysis?
Specifically, is the schedule itself a cause of arrhythmias?
If arrhythmias were to occur uniformly during the calendar week, one would be hard-pressed to argue that the conventional hemodialysis schedule is a culprit. However, uniformity of timing is far from apparent.
Clinically significant arrhythmias are clustered around the first hemodialysis session of the week and at the ends of every interdialytic gap, including the long gap. These are the hours when the fluid load is highest, when the serum potassium concentration is highest, and specifically during the first hemodialysis session of the week, when the ultrafiltration rate is likely highest.2 Remarkably, bradycardia is a hallmark of the ends of every interdialytic gap, short or long.
The MiD Study sadly shows that for many thrice-weekly hemodialysis patients, normal heart rhythm is not maintained. Effective solutions to this problem are not easy to identify. Extending session length mathematically lowers the ultrafiltration rate and may lower the risk of clinical significantly arrhythmias and atrial fibrillation during hemodialysis sessions; extending session length during the first session of the week may be especially helpful.
However, unless treatment frequency is increased, each calendar week is still characterized by two short (48-hour) gaps and one long (72-hour) gap between consecutive dialysis sessions. Increasing treatment frequency is the only way to reduce the length of these gaps. Thus, increasing treatment frequency may be the most direct method to reduce risk of arrhythmias. However, without a randomized clinical trial of hemodialysis frequency in patients with implantable, continuous cardiac monitoring devices, we do not know that increasing treatment frequency is efficacious.
Importantly, it is possible that the incidence of arrhythmias on thrice-weekly hemodialysis can be reduced with adjustments to dialysate temperature, dialysate composition, and use of oral medications that alter heart rate and rhythm. Use of oral potassium binders may also be helpful.4
To be certain, sudden cardiac death is common in the dialysis patient population. Addressing the high incidence of arrhythmias should be of paramount concern. More studies and more clinical strategies are urgently needed.
Available Downloads
Potential Risks
Not everyone will experience the reported benefits of home and more frequent hemodialysis. All forms of hemodialysis involve some risks. When vascular access is exposed to more frequent use, infection of the site, and other access related complications may also be potential risks.
Certain risks associated with hemodialysis treatment are increased when performing solo home hemodialysis because no one is present to help the patient respond to health emergencies.
Certain risks associated with hemodialysis treatment are increased when performing nocturnal therapy due to the length of treatment time and because the patient and care partner are sleeping.
