Better Management of Volume with Intensive Hemodialysis
In the March issue of Nephrology News & Issues, I described the challenge of managing volume during and between hemodialysis sessions.
The root of the challenge is the intermittent nature of hemodialysis.
In the dominant schedule, intermittency is marked by 3 hemodialysis sessions per week, either on Monday-Wednesday-Friday or Tuesday-Thursday-Saturday, for 210 to 230 minutes per session.
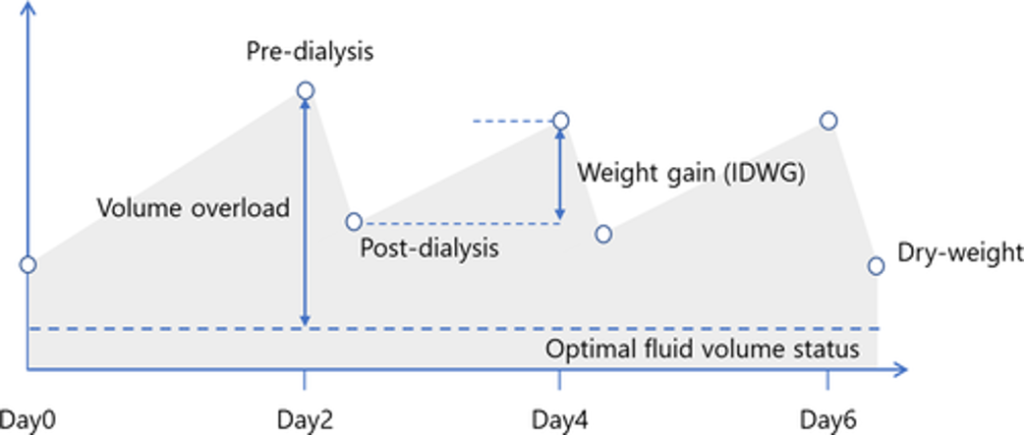
The implication of this schedule is that we have 3 interdialytic gaps per week, including one gap of approximately 68 hours, during which fluid accumulates in the body, and we have all of 11 hours during which the hemodialysis machine removes that fluid.1
Accumulating Volume Between Sessions
During each interdialytic gap, fluid accumulates in the extracellular space.
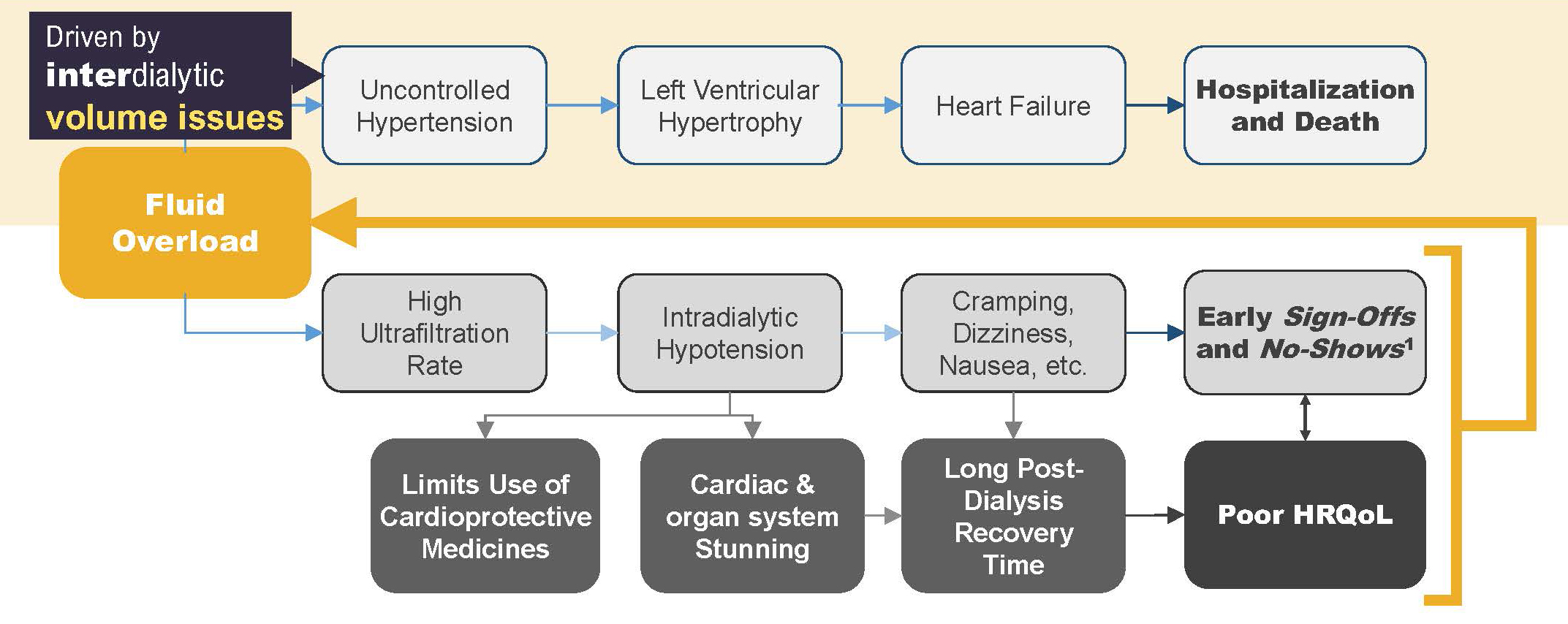
This volume expansion can quickly lead to the development of uncontrolled hypertension, as defined by either elevated systolic blood pressure or normal systolic blood pressure accompanied by use of 3 or more antihypertensive medications (e.g., the trio of lisinopril, metoprolol, and amlodipine).2
Persistent hypertension leads to the development of left ventricular hypertrophy and eventually clinically evident heart failure, which is associated with increased rates of hospitalization and death.3,4
Zoccali and colleagues recently showed that a state of chronic fluid overload, as measured by bioimpedance spectroscopy, is associated with increased risk of death, regardless of whether pre-dialysis systolic blood pressure is normal or elevated.5
The long interdialytic gap at the end of each week is well-established in multiple registry studies around the world as a risk factor for cardiovascular hospitalization and sudden cardiac death.6
More recently, implantable loop recorders have shown that the long interdialytic gap is also associated with increased risk of bradycardia.7
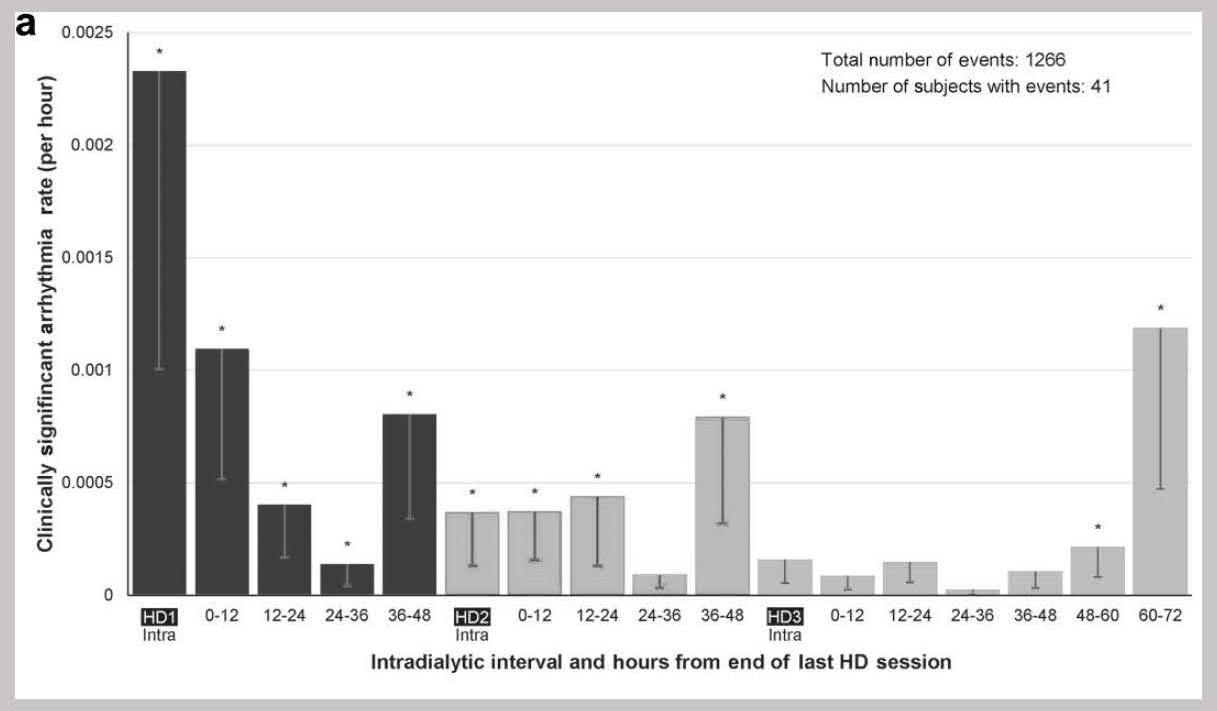
These data collectively suggest that there is much to be gained by ensuring normal volume status in patients on intermittent hemodialysis.
Removing Volume During Sessions
The first step in ensuring that status is removing fluid when the opportunity exists. In other words, ultrafiltration during all available hemodialysis hours is essential. However, we have learned during the past decade that aggressive ultrafiltration comes at a cost.8
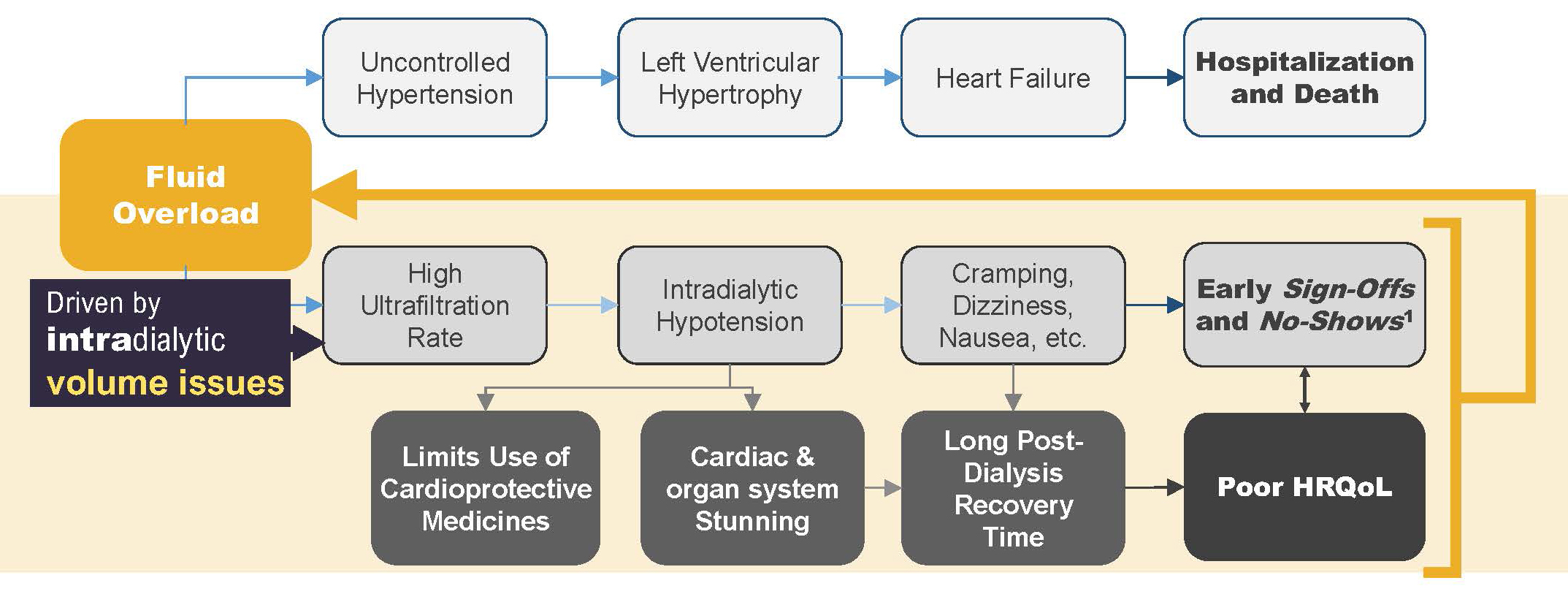
A very high ultrafiltration rate is likely to lead to acute volume depletion, thus resulting in intradialytic hypotension, the symptoms of which include cramping, dizziness, and nausea.9 Patients who experience these symptoms may choose to “sign off” treatment early, if not to skip treatment entirely.10 Intradialytic hypotension is also associated with cardiac and other organ stunning, which may be responsible for the very long post-treatment recovery times that many patients report.11,12
Slower Ultrafiltration is Better
Observational studies show that higher ultrafiltration rates, scaled to body weight, are strongly associated with risk of death.13 One study of 118,394 in-center hemodialysis patients reported an incremental association between ultrafiltration rate and risk of death, when the ultrafiltration rate exceeded 8 mL/hour/kg.14
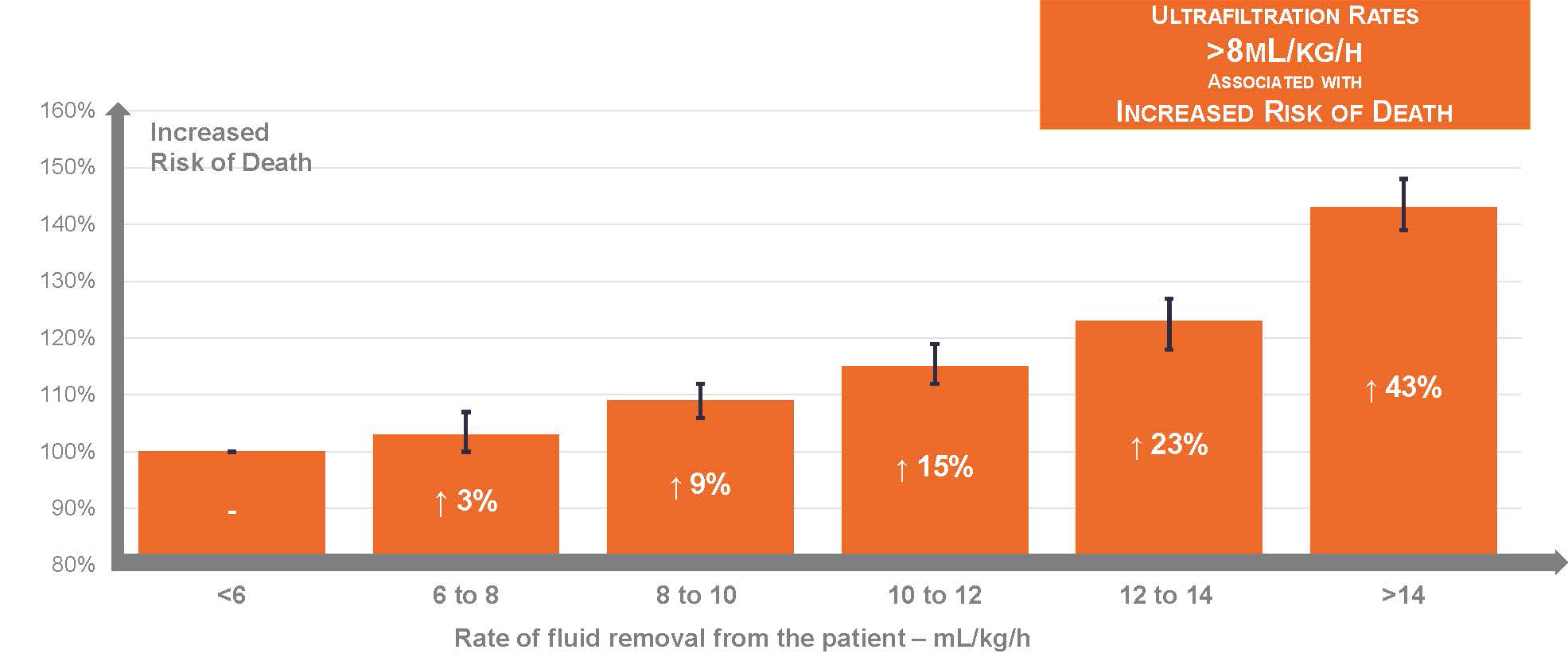
In another study of patients receiving longer hemodialysis sessions (i.e., median duration of 5 hours), Chazot and colleagues showed that even an ultrafiltration rate exceeding 6.8 mL/hour/kg was associated with 90% higher risk of death.15
In other words, slower ultrafiltration is better.
Recent Emphasis on Ultrafiltration Intensity Appears to Have Made Its Mark
Let’s list the goals:
- Always maintain normal volume status
- Avoid long interdialytic gaps
- Slowly remove accumulated fluid with ultrafiltration
We are making progress on the third goal. During the past decade, the ultrafiltration rate among in-center hemodialysis patients has steadily declined.16
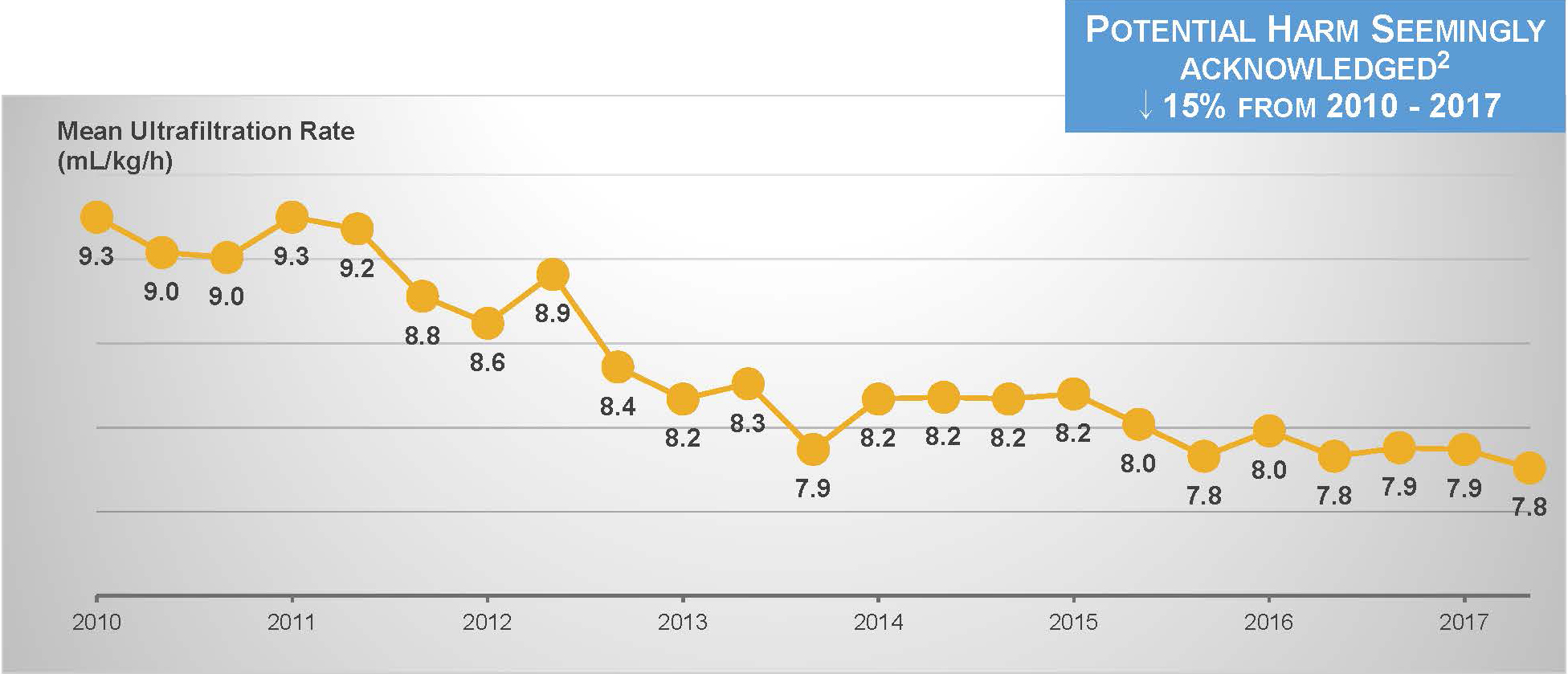
However, nothing else has changed.
Hemodialysis frequency is certainly unchanged and session duration has increased only by a few minutes.16
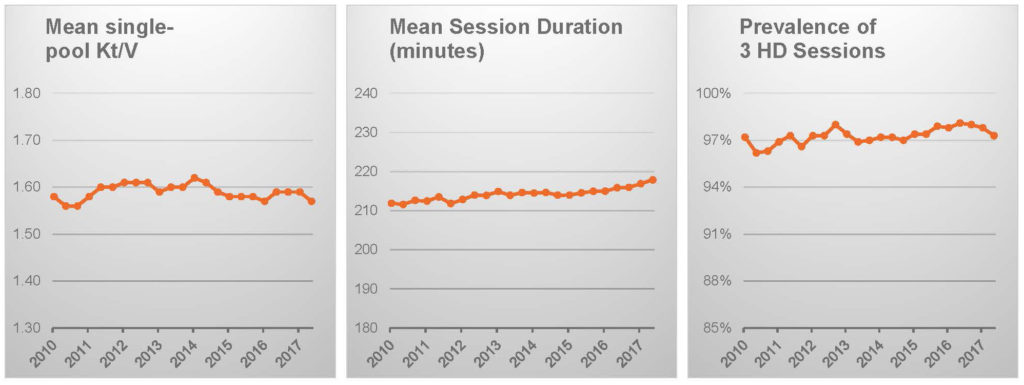
Patient characteristics, notably including body weight, appear to be stable.16
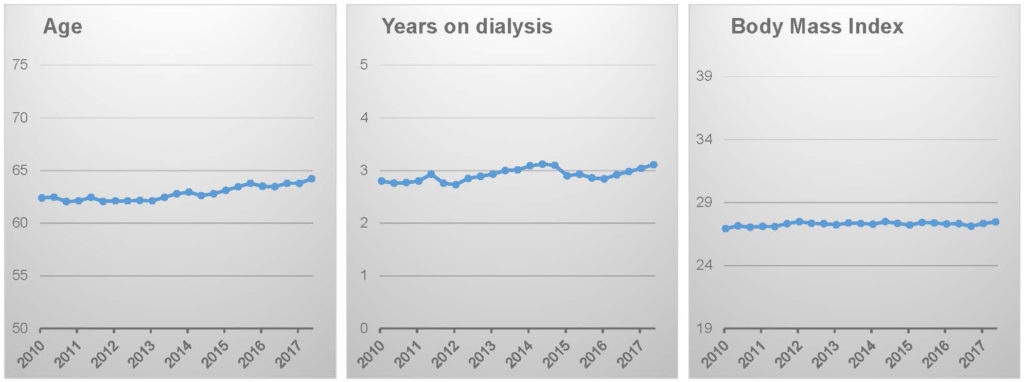
The logical conclusion, which is supported by ecological analysis of DOPPS Practice Monitor data, is that targeting a lower ultrafiltration rate has come at the expense of limiting ultrafiltration volume (i.e., intradialytic weight loss), thus leaving patients in a relatively “wetter” state than in the past.16
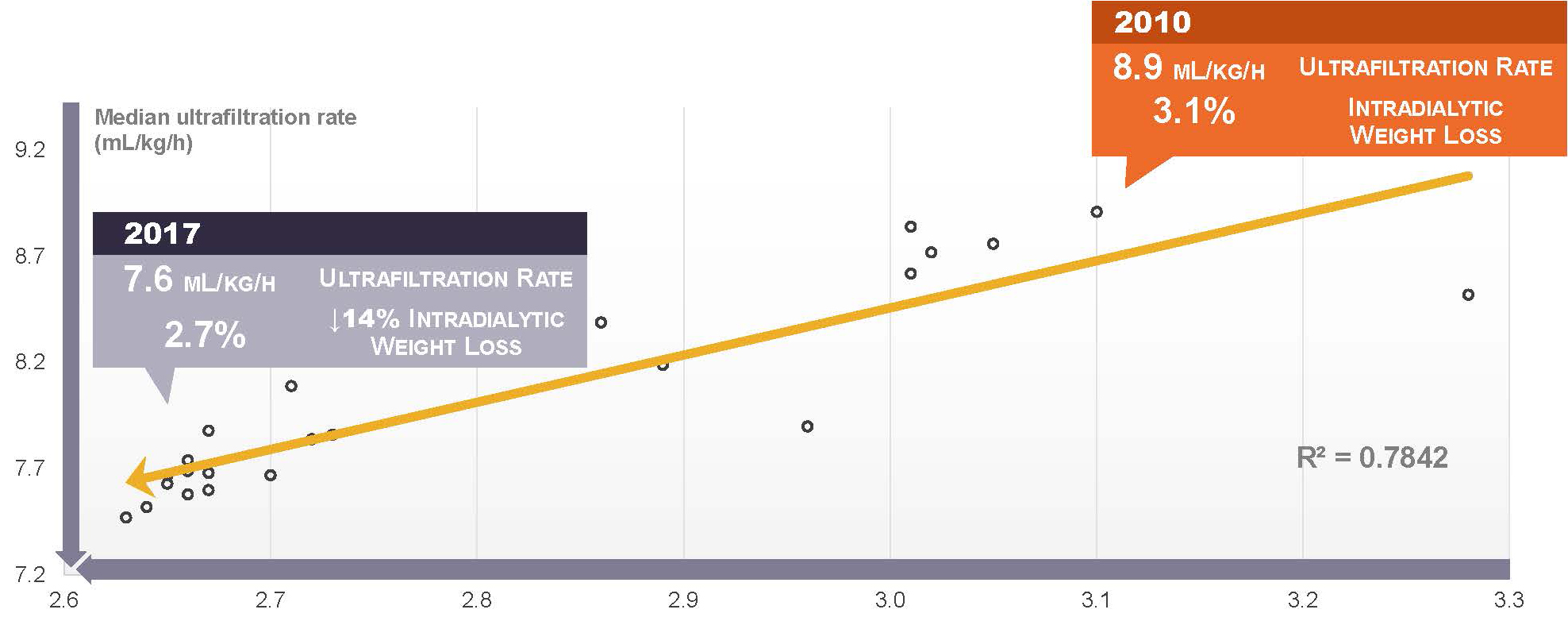
In other words, we have lowered the ultrafiltration rate, but kept the long interdialytic gap, and probably retreated from normal volume status.
What is the net effect?
Unfortunately, new data from the United States Renal Data System indicates that the rate of cardiovascular hospitalization has suddenly turned upward.17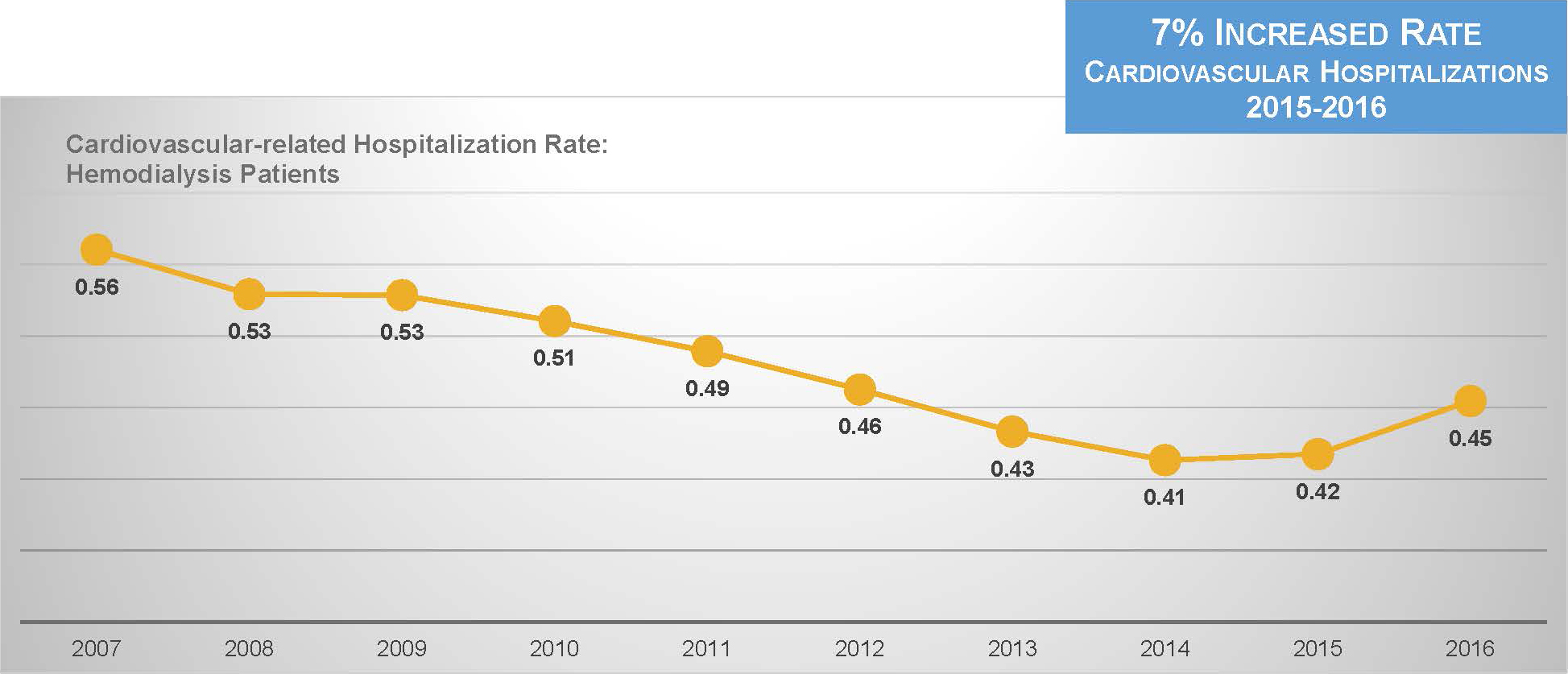
This is predictable. Multiple studies have shown that positive differences between post-dialysis weight and estimated dry weight are associated with worse clinical outcomes.5,18,19

The Solution: Remove the Treatment Constraints
Given the constraints of 3 sessions and 11 cumulative hemodialysis hours per week, there is not a lot that can be done, short of arresting fluid intake between treatments. With a relatively salt-laden food supply in the United States, limiting fluid intake is obviously not easy.20 We need not continue to impose constraints on treatment.
To advance practice, we need to relax the operational constraint imposed by shift-based hemodialysis in the facility setting. In the home setting, in which hemodialysis intensity can be customized to the needs of the patient, the constraints are lifted.
Emerging data clearly demonstrate the consequences.
Interdialytic weight gain on frequent hemodialysis
In an elegant post hoc analysis of the Frequent Hemodialysis Network trials, Raimann and colleagues showed that time-integrated fluid load was sharply reduced with 6 versus 3 sessions per week.21
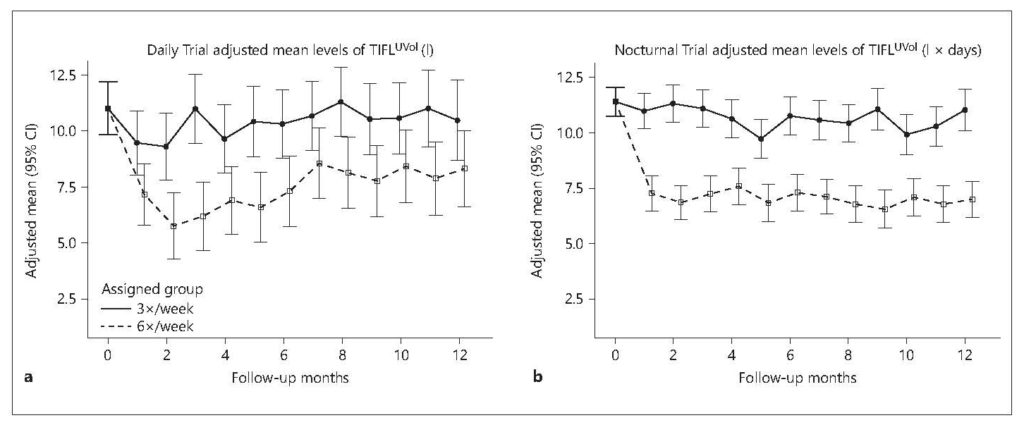
The mathematics of the study are complex, but the observation is truly a simple one: with increased treatment frequency, there is less time between consecutive treatments, so fluid has less time to pool in the extracellular space.
Ultrafiltration intensity on frequent diurnal hemodialysis
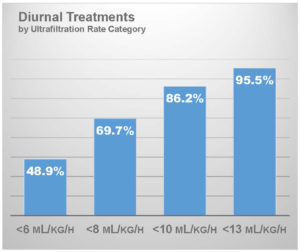
An increasing volume of home hemodialysis patients in the United States use a connected health platform that collects data about every hemodialysis session, including session duration and ultrafiltration volume. These data can be used to characterize the ultrafiltration intensity on frequent home hemodialysis. In an analysis of digital flowsheets that have been collected through January 2019, I have characterized the ultrafiltration rate in patients who have been prescribed at least 4 sessions per week and who dialyze during daytime hours (N = 84,128 patient-weeks).22
With an average session duration of 174 minutes, mean ultrafiltration rate was 6.5 mL/hour/kg. Roughly 49% of patients had an ultrafiltration rate below 6 mL/hour/kg and importantly, 86% had an ultrafiltration rate below 10 mL/hour/kg. This is a salient observation for patients with heart failure, as post hoc analysis of the HEMO trial showed that an ultrafiltration rate exceeding 10 mL/hour/kg was associated with increased risk of death in this subgroup.8
Ultrafiltration intensity on frequent nocturnal hemodialysis
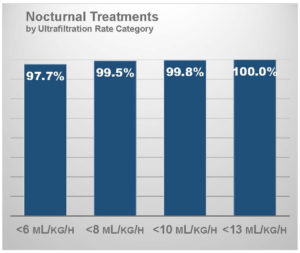
In a similar analysis, I have characterized the ultrafiltration rate in patients who have been prescribed at least 4 sessions per week and who dialyze during nighttime hours (N = 7,757 patient-weeks).22 With an average session duration of 425 minutes, thereby resulting in over 27 treatment hours per week, mean ultrafiltration rate was only 3.0 mL/hour/kg.
Over 97% of patients had an ultrafiltration rate below 6 mL/hour/kg and nearly all patients had an ultrafiltration rate below 10 mL/hour/kg.
Frequency and duration make all the difference in hemodialysis
We can positively address volume loading between hemodialysis sessions and ultrafiltration intensity by increasing treatment frequency and cumulative treatment hours per week.
This is nothing more than simple arithmetic.
Translating Science into Practice
We have a deepening understanding that limiting ultrafiltration intensity is an important goal, as excessive intensity leads to organ stunning and myocardial ischemia. However, as we resolve to limit the ultrafiltration rate, we cannot inadvertently allow fluid to accumulate in hemodialysis patients, thereby resulting in an unintended epidemic of volume and pressure overload.
Ultimately, we can try to limit fluid intake. We can even exploit technology (e.g., bioimpedance spectroscopy and lung ultrasonography) to better estimate dry weight and mitigate the risk of otherwise unrecognized chronic fluid overload. However, these tactics probably permit only limited gains. If the usual treatment schedule itself creates risk, by leaving long gaps between treatments and compressing ultrafiltration demand into a limited number of hours per week, we cannot attain a state that more closely resembles normal volume regulation.
Better management of volume can be achieved with intensive hemodialysis.
By increasing treatment frequency, we can shorten the length of interdialytic gaps, thus limiting the total fluid load. Data from current home hemodialysis patients in the United States show that frequent diurnal hemodialysis lowers ultrafiltration intensity below the norm of in-center hemodialysis, and that frequent nocturnal hemodialysis bests even its diurnal counterpart.
This is a win-win scenario.
Available Downloads
Potential Risks
Not everyone will experience the reported benefits of home and more frequent hemodialysis. All forms of hemodialysis involve some risks. When vascular access is exposed to more frequent use, infection of the site, and other access related complications may also be potential risks.
Certain risks associated with hemodialysis treatment are increased when performing solo home hemodialysis because no one is present to help the patient respond to health emergencies.
Certain risks associated with hemodialysis treatment are increased when performing nocturnal therapy due to the length of treatment time and because the patient and care partner are sleeping.
