Recasting Kidney Failure as Cardiovascular Disease
Total Medicare expenditures on the health care of patients with permanent kidney failure have steadily risen to more than $35 billion per year, although per capita expenditures on the care of dialysis patients have been relatively stable in recent years.1 This conflict in trends can be readily explained by the seemingly unstoppable increase in the number of people undergoing chronic dialysis.
Dialysis Patient Population Growth
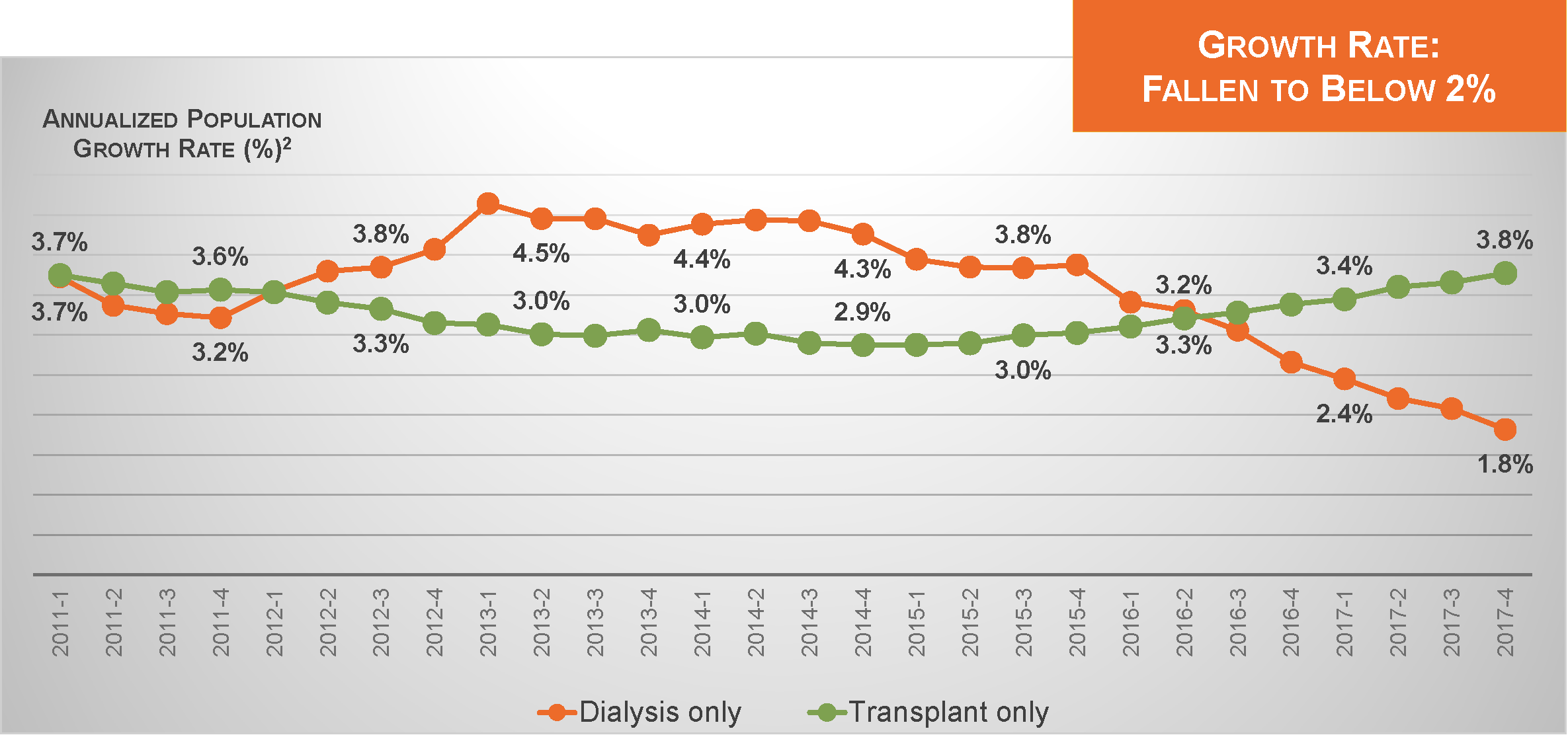
Today, that number exceeds 510,000. Nevertheless, the annualized rate of growth in the dialysis patient population has recently slipped below 2%.2 This slowdown provides an excellent opportunity to reassess the role of dialysis in the landscape of U.S. health care.
For decades, dialysis has been understood as treatment for uremia and hypervolemia. From the perspective of clearing urea and removing fluid, dialysis can be delivered in the form of either hemodialysis or peritoneal dialysis, in either a health care facility or a private residence. In fact, decisions about modality and setting can seem trivial, as solute diffusion and ultrafiltration can occur under any number of dialytic circumstances. As a result, the home setting may be viewed positively simply because of lower cost.
Home Dialysis Utilization
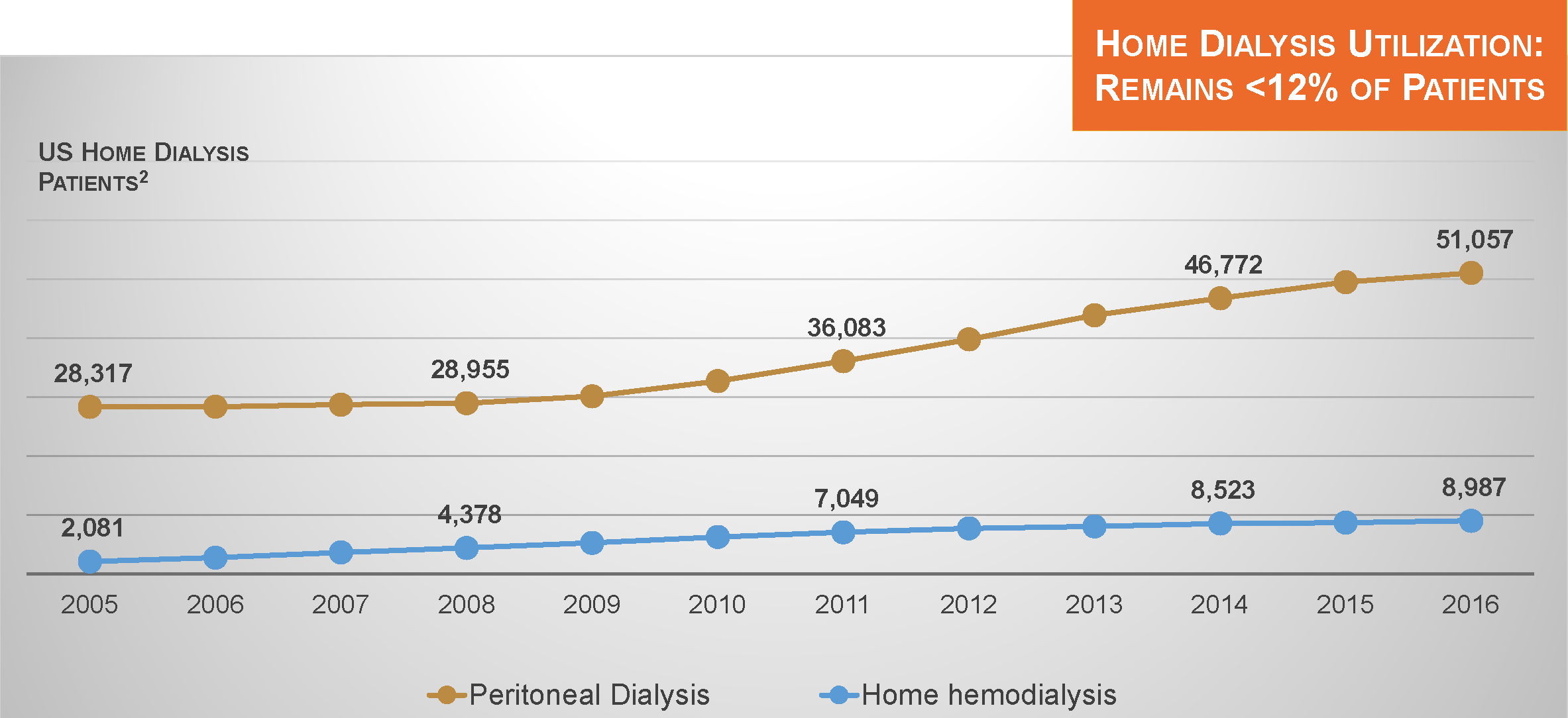
In recent years, utilization of both peritoneal dialysis and home hemodialysis has increased in the US. However, despite progress, these modalities are utilized by only 12% of dialysis patients.2
Is 12% a “good” level of utilization? That is a difficult question to answer. Perhaps the better question to ask is this: are contemporary outcomes in the dialysis patient population “good”? Many press releases have touted the significant improvement in dialysis patient survival occurring between roughly 2001 and 2013. Unfortunately, since 2013, there has been little evidence of further progress.
Hospitalization Utilization
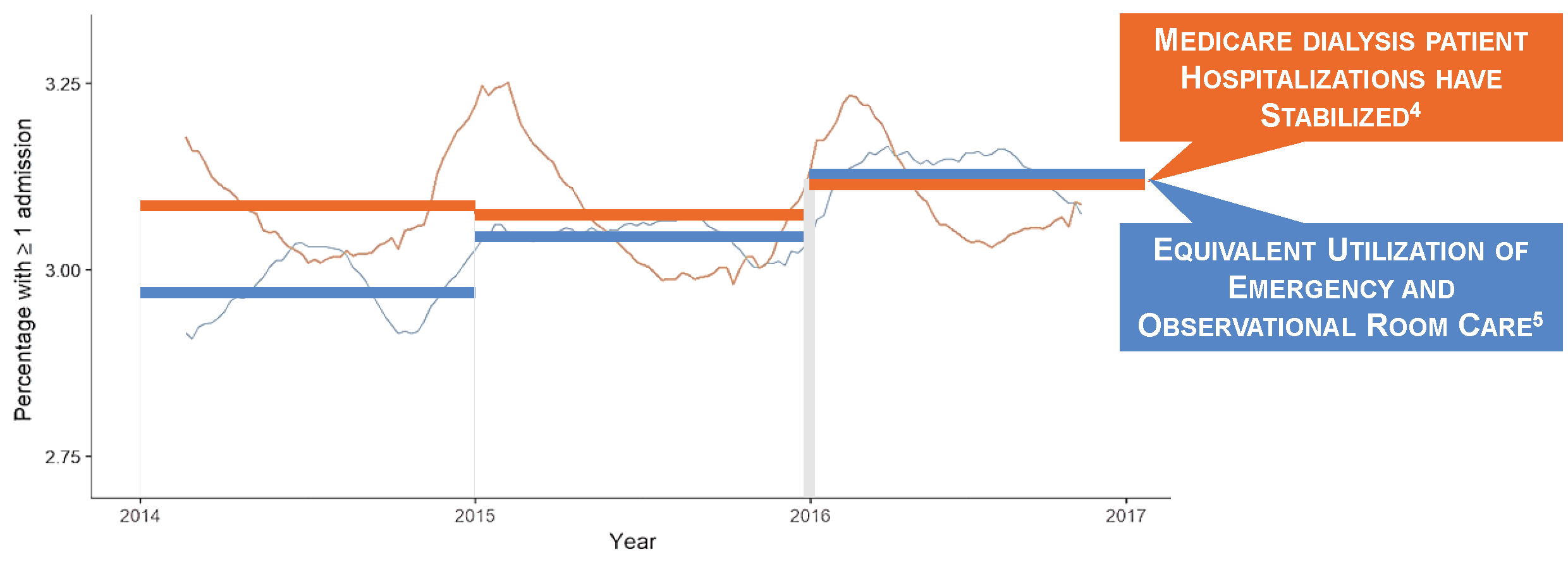
Both death and hospital admission rates were flat between 2014 and 2016.3,4 Encounter rates in the emergency and observation rooms have risen to the point that weekly risks of a new hospital admission or an outpatient encounter in the emergency or observation room are roughly equal.4,5 Interestingly, during periods of progress and stagnation alike, it is cardiovascular disease that has remained the leading cause of death.6 We should not be surprised. Consider how differently we approach volume management in dialysis patients and non-dialysis-dependent patients with chronic kidney disease.
Volume Management
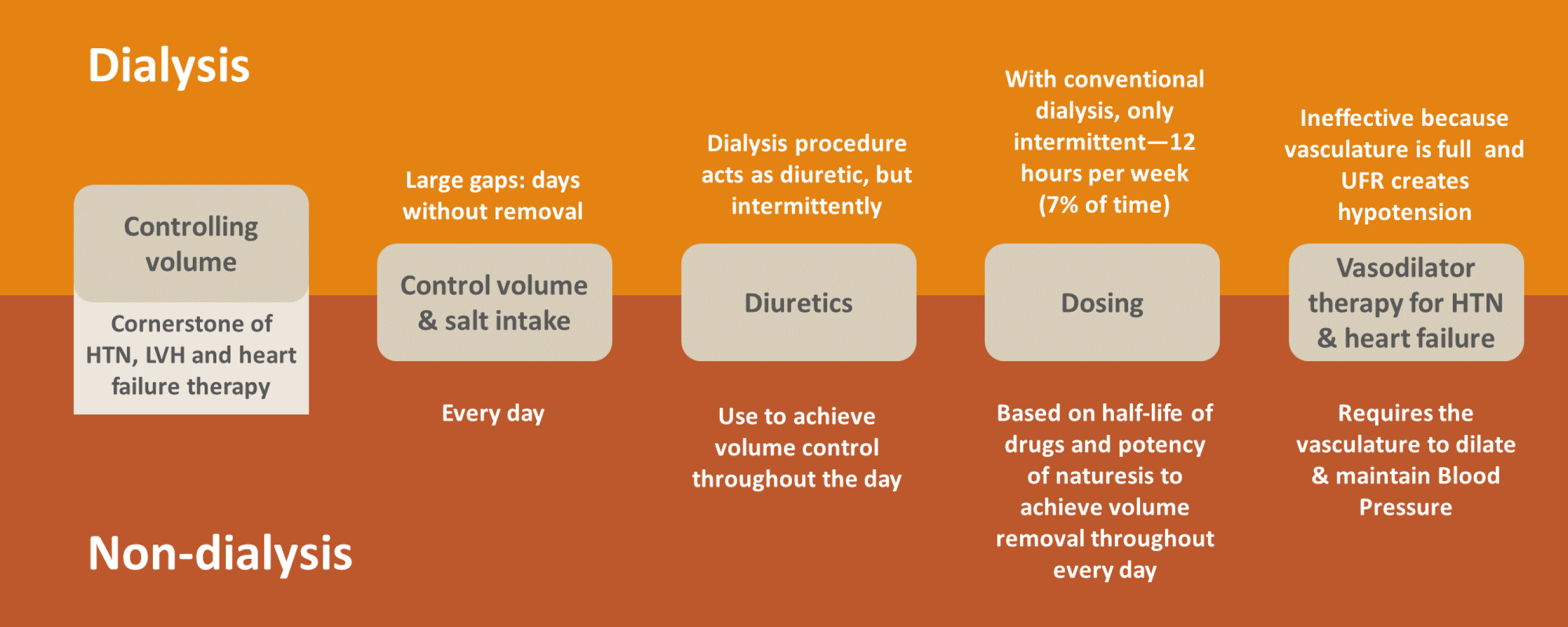
In the latter group, patients have ample endogenous kidney function and we prescribe diuretics to increase urine output. Quite literally, excess fluid is removed every hour. In dialysis patients, we typically rely on intermittent therapy, with gaps ranging from 48 to 72 hours between consecutive rounds of ultrafiltration. With 4-hour hemodialysis sessions, excess fluid is removed during only 7% of the hours in a week. In this view, kidney failure can be characterized as a state of persistent fluid overload.
Causal Path of Cardiovascular Disease
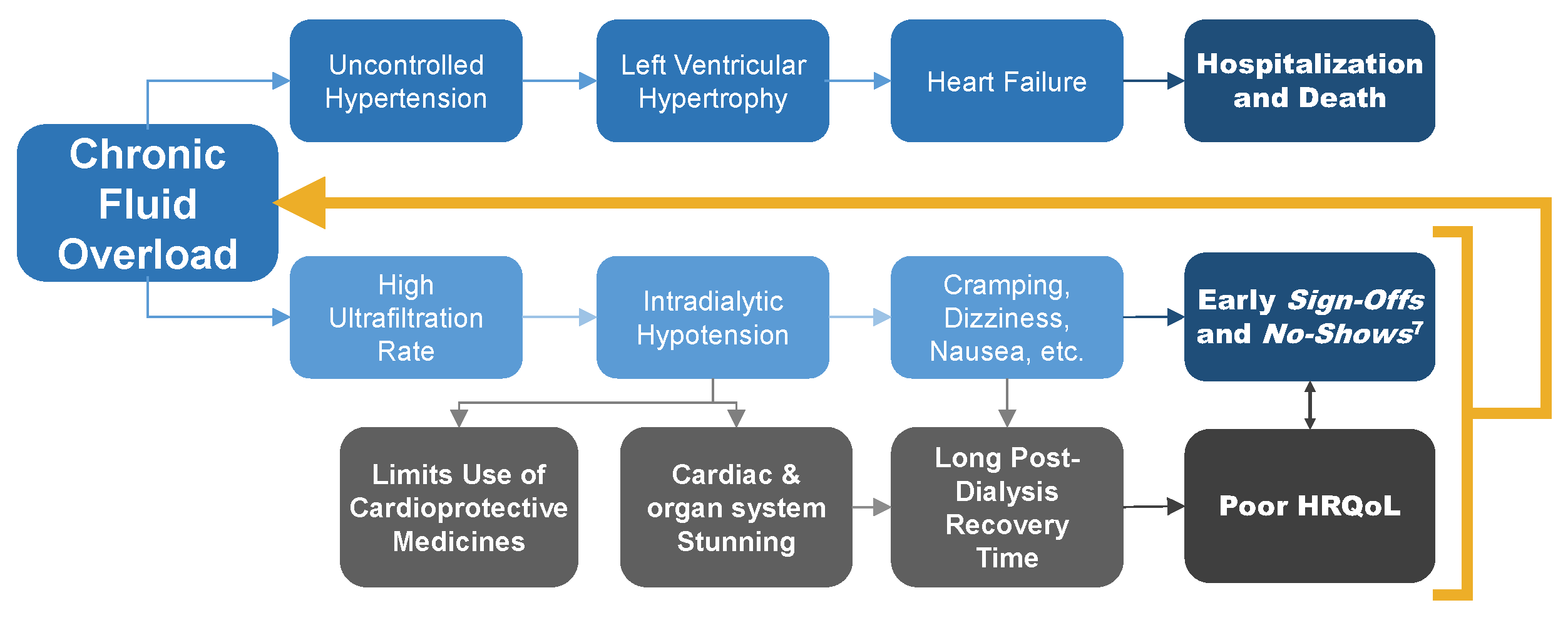
Chronic fluid overload leads to a cascade of uncontrolled hypertension, left ventricular hypertrophy, and heart failure.7 In turn, we observe frequent arrhythmias and high incidence of sudden cardiac death.8,9,10 Attempts at compressing weekly ultrafiltration needs into 12 hours of hemodialysis lead to intradialytic hypotension and cardiac stunning.11
In turn, patients experience negative symptoms, including cramping, dizziness, and nausea during treatment and long recovery time after treatment. These problems diminish quality of life, increasing the risk of abbreviated and missed treatments and further reinforcing the state of persistent fluid overload.7,12
Looking at kidney failure in this way—as a state of persistent fluid overload—means that we recast the disease, looking at it not as uremia, but as a predominantly cardiovascular disease. Coincidentally, this recasting of kidney failure comes while SGLT2 inhibitors have shifted the pharmacologic treatment of diabetes mellitus in non-dialysis-dependent chronic kidney disease from targeting glycated hemoglobin to targeting cardiovascular risk!
From this vantage point, we must then ask whether the dominant dialytic modality of thrice-weekly in-center hemodialysis is an effective therapy. Evidence from multiple studies paints a sobering picture. Chronic fluid overload is present in roughly 30% of hemodialysis patients and associated with a markedly elevated risk of death.13 The long interdialytic gap between Friday and Monday (or Saturday and Tuesday) is also associated with elevated risks of cardiovascular death and hospitalization.14 The prevalence of hypertension, as defined by pre-dialysis systolic blood pressure greater than 140 mm Hg, is 60% in US dialysis patients, despite widespread use of multiple antihypertensive agents.15 Recent observational studies show that every increment in the rate of ultrafiltration, even at the lower end of the gradient, is associated with an increased risk of death.16 Clearly, it is challenging to adequately treat cardiovascular disease with intermittent hemodialysis.
Therapeutic alternatives
We know from multiple randomized clinical trials and large observational cohort studies that hemodialysis for 5 or 6 sessions per week can positively address apparent pathophysiology. Frequent hemodialysis reduces pre-dialysis systolic blood pressure, lessens the need for antihypertensive medications, and lowers left ventricular mass.17,18,19 Relative to both thrice-weekly in-center hemodialysis and peritoneal dialysis, frequent hemodialysis is associated with significantly lower risk of cardiovascular hospitalization.20,21 It reduces serum phosphorus, a risk factor of vascular calcification, and finally, frequent hemodialysis dramatically shortens recovery time after treatment.22,23
Frequent hemodialysis can be prescribed for both reactive and proactive care. In the former scenario, candidates for frequent hemodialysis comprise patients with persistently uncontrolled hypertension, left ventricular hypertrophy, advanced heart failure, chronic hyperphosphatemia, or refractory hypervolemia on peritoneal dialysis.24 In the latter, candidates include patients awaiting a kidney transplant, pregnant women, and young adults who aim to devote daytime hours to activities unrelated to dialysis.24
Recasting kidney failure as cardiovascular disease is key to recapturing our past momentum toward better outcomes among dialysis patients. Increasing hemodialysis directly addresses the state of persistent fluid overload that engenders cardiovascular disease. Frequent hemodialysis can be used for either secondary or primary prevention of cardiovascular disease, given careful evaluation and selection of patients. It is time to give the therapy greater consideration. We owe this to current and future patients entrusted to our care.
Available Downloads
Download Slides | Recent Findings: Recasting Kidney Failure as Cardiovascular Disease
Potential Risks
Not everyone will experience the reported benefits of home and more frequent hemodialysis. All forms of hemodialysis involve some risks. When vascular access is exposed to more frequent use, infection of the site, and other access related complications may also be potential risks.
Certain risks associated with hemodialysis treatment are increased when performing solo home hemodialysis because no one is present to help the patient respond to health emergencies.
Certain risks associated with hemodialysis treatment are increased when performing nocturnal therapy due to the length of treatment time and because the patient and care partner are sleeping.
